Abstract
Background: Polycythaemia vera (PV) is characterised by thrombosis, hemorrhage, systemic symptoms and disease transformation. In high-risk PV ruxolitinib controls blood counts, improves symptoms, but efficacy for thrombosis and transformation endpoints is uncertain. Furthermore the clinical benefit of reduction of JAK2V617F variant allele fraction (VAF) is also unproven.
Methods: MAJIC-PV is a randomized phase II trial of ruxolitinib vs Best Available Therapy (BAT) in patients resistant/intolerant to hydroxycarbamide (HC-INT/RES) with no cross-over between arms. Primary outcome was complete response (CR) per European LeukemiaNet criteria within 1 year. Secondary outcomes included duration of response, event-free survival, symptom and molecular response.
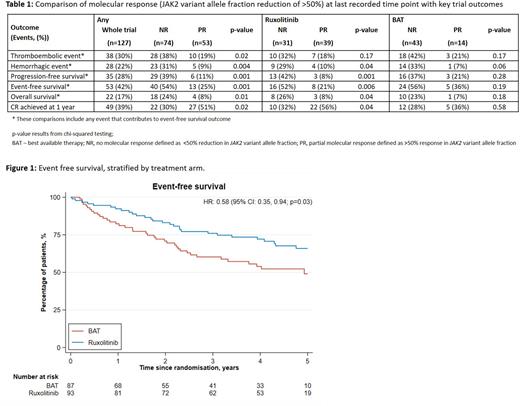
Results: Overall 180 patients were randomized. At 1 year CR was achieved in 40 (43%) patients on ruxolitinib vs 23 (26%) on BAT (OR 2.12, 90% CI 1.25, 3.60, p=0.02). The median treatment duration on ruxolitinib was 1568 days and 1220 days for BAT patients. Duration of CR was superior for ruxolitinib (HR 0.38, 95% CI 0.24, 0.61, p<0.001). Symptom responses were better with ruxolitinib and durable to a mean of 52 months. Thromboembolic-event free, but not hemorrhage-free, survival was significantly improved with ruxolitinib (HR 0.56, 95% CI 0.32, 1.00, p=0.05) and correlated with average number of venesections per year (HR 1.20, 95% CI 1.08, 1.33, p<0.001). Event-free (major thrombosis, hemorrhage, transformation and death) survival (EFS) was superior for patients attaining CR within 1 year (HR 0.41, 95% CI 0.21, 0.78, p=0.01); and those on ruxolitinib (HR 0.58, 95% 0.35, 0.94, p=0.03) (Figure 1). Serial analysis of JAK2V617F VAF revealed molecular response (per ELN criteria), ie >50% VAF reduction, at 12m was only observed in 14% (9 of 63) ruxolitinib and 18% (9 of 50) BAT patients respectively and was more common in those in CR at 1 year. Over time molecular responses deepened thus at the last time point tested, a >50% reduction observed in 56% (39/70) and 25% (14/57), of ruxolitinib and BAT respectively (p<0.001). Furthermore this molecular response was associated with improved outcomes (progression-free survival p=0.001, EFS p=0.001, overall survival p=0.01, (Table). To analyze clonal burden in stem/progenitor cells (HSPC) targeted single-cell genotyping was performed on three ruxolitinib treated patients were selected confirming substantial (72 - 100%) reduction in JAK2V617F+ HSPC. Individuals with gene panel sequencing and time-to-event data were dichotomized into single versus ≥2 driver mutations. Survival analysis demonstrated impaired EFS in patients with additional driver mutations (treatment, age and sex-adjusted HR=1.92, 95% CI 1.16, 3.19, n=167, p=0.01). Specifically, mutated-ASXL1 conferred impaired EFS (adjusted HR 3.02, 95% CI 1.47, 6.17, n = 167, p = 0.003) correcting for age, gender and the presence of mutations in TET2. The safety profile of ruxolitinib was as previously reported.
Conclusions: Overall, MAJIC-PV demonstrates novel benefits for ruxolitinib which improved thrombosis-free and EFS in high risk HC-int/res PV; whilst confirming existing evidence of efficacy for hematological control and symptom responses. Importantly, embedded pre-planned analyses within this study demonstrate for the first time that attainment of a 50% reduction in JAK2V617F VAF, which occurred more frequently with ruxolitinib, was associated with important clinical benefits (attaining CR, improved PFS, EFS, OS) and clearance of MPN stem cells. This data confirms and challenges the current therapeutic algorithm, supporting the benefit of targeted therapy and molecular monitoring in this disease.
Disclosures
Harrison:Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Promedior: Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau; Galacteo: Membership on an entity's Board of Directors or advisory committees; Sierra: Honoraria; Shire: Membership on an entity's Board of Directors or advisory committees; Geron: Membership on an entity's Board of Directors or advisory committees; AOP Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Membership on an entity's Board of Directors or advisory committees, Research Funding; Galecto: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Keros: Consultancy; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings, Research Funding; EHA: Other: Leadership role; MPN voice: Other: Leadership role; Gilead: Membership on an entity's Board of Directors or advisory committees. Yap:Faron Pharmaceuticals: Other: Personal fees. Mesa:Promedior: Research Funding; Genotech: Research Funding; Samus: Consultancy, Research Funding; AbbVie: Research Funding; CTI: Research Funding; AOP: Consultancy; Bristol Myers Squibb: Consultancy; Incyte: Consultancy, Research Funding; Celgene: Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Research Funding; Roche: Consultancy; LaJolla Pharmaceutical: Consultancy; Sierra Oncology: Consultancy, Research Funding; Blueprint: Consultancy; Geron: Consultancy; Novartis: Consultancy; Gilead: Research Funding; Imago: Research Funding. Green:Novartis: Other: Personal fees for presentation at multiple MPN preceptorships. Coppell:Novartis: Other: Sponsorship to attend EHA 2021 meeting. Garg:Janssen, Takeda, Navartis, Amgen, BMS, GSK: Consultancy, Honoraria; Janssen, Amgen, Takeda, Novartis: Consultancy, Other: Ad Board; Janssen, Amgen: Consultancy, Speakers Bureau. Ewing:Novartis: Other: Speaker meeting honoraria. Knapper:Novartis: Research Funding, Speakers Bureau; Servier: Consultancy, Honoraria; BMS: Consultancy; Astellas: Speakers Bureau; Jazz: Consultancy, Honoraria, Speakers Bureau. Godfrey:AOP Orphan: Other: Personal fees; Celegene: Other: Personal fees; Novartis: Other: Personal fees. Drummond:Blueprint Medicines Corporation: Research Funding; Novartis: Other: Personal fees, Research Funding. Byrne:Novartis: Honoraria, Speakers Bureau. McMullin:CTI: Consultancy; AOP: Research Funding, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Sierra Oncology: Consultancy; Incyte: Consultancy, Speakers Bureau; BMS: Consultancy, Research Funding; Novartis: Consultancy, Speakers Bureau; Pfizer: Speakers Bureau. Mead:AbbVie: Consultancy, Speakers Bureau; Celgene/BMS: Consultancy, Research Funding, Speakers Bureau; Sensyn: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Galecto: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Alethiomics Ltd: Consultancy, Current equity holder in private company, Other: Co-founder and equity holder, Research Funding; Karyopharm: Consultancy, Speakers Bureau; Sierra Oncology: Consultancy, Speakers Bureau; CTI: Consultancy, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal